- Research
- Open access
- Published:
Effects of simulated smoke condensate generated from combustion of selected military burn pit contents on human airway epithelial cells
Particle and Fibre Toxicology volume 21, Article number: 41 (2024)
Abstract
Background
Exposure to military burn pit smoke during deployment is associated with different respiratory and non-respiratory diseases. However, information linking smoke exposure to human pulmonary health is lacking. This study examined the effects of simulated burn pit smoke condensates on human airway epithelial cells (HAECs) from twelve donors (smokers/non-smokers, biological female/male) cultured at an air-liquid interface and exposed to condensates from three simulated burn pit waste materials (cardboard, plywood, and plastic) incinerated at two combustion conditions: smoldering and flaming. Cellular gene expression was analyzed using bulk RNA sequencing, and basolateral media cytokine levels were assessed using multiplex immunoassay.
Results
Flaming smoke condensates caused more significant differentially expressed genes (DEGs) with plywood flaming smoke being the most potent in altering gene expression and modulating cytokine release. Cardboard and plywood flaming condensates primarily activated detoxification pathways, whereas plastic flaming affected genes related to anti-microbial and inflammatory responses. Correlation analysis between smoke condensate chemicals and gene expression to understand the underlying mechanism revealed crucial role of oxygenated polycyclic aromatic hydrocarbons (PAHs) and aluminum, molybdenum, and silicon elements; IL6 expression was positively correlated with most PAHs. Stratification of data based on HAEC donor demographics suggests that these affect gene expression changes. Enrichment analysis indicated similarity with several deployment-related presumptive and reported diseases, including asthma, emphysema, and cancer of different organs.
Conclusions
This study highlights that simulated burn pit smoke exposure of HAECs causes gene expression changes indicative of deployment-related diseases with more pronounced effects seen in smokers and females. Future studies are needed to further characterize how sex and smoking status affect deployment-related diseases.
Introduction
During military operations in Central and Southwest Asia, Africa, and other locations, deployed personnel experienced various inhalation exposures from environmental and anthropogenic sources [1]. The mean ambient concentration of particulate matter (PM) in these areas was frequently more than exposure limits suggested by National Ambient Air Quality Standards and the US Environmental Protection Agency [1]. Deployed personnel returning from the Persian Gulf war in the 90s reported development of pulmonary symptoms, which led to the US Department of Defense’s implementation of the Enhanced Particulate Matter Surveillance Program (EPMSP) [2]. EPMSP reported three major sources of PM exposure to deployed veterans: geologic dust, burn pit smoke, and heavy metal condensates [1, 3]. Burn pits were a common means of solid waste management strategy practiced during the military operations which involved open air burning of various solid wastes, resulting in the generation of toxic chemical compounds, including polychlorinated dibenzo-p‐dioxins and dibenzofurans (PCDD/Fs), polyaromatic hydrocarbons (PAHs), volatile organic compounds (VOCs), metals, and PMs [4]. Consequently, deployment-related exposure has been linked with several respiratory diseases, including chronic obstructive pulmonary disease (COPD), asthma, bronchiolitis, lung cancer and interstitial lung diseases [5,6,7,8]. Several open burning facilities are still active within the US [9] and worldwide [10] contributing and aggravating the environmental pollution level and posing a significant public health hazard. In fact, similar toxic emission from municipal solid waste incineration has been a major concern for decades [11,12,13].
Several analyses support the perception that post-deployment respiratory and other health effects are at least partly attributable to burn pit smoke exposure. In a cross-sectional study Garshick et al. analyzed data from the Service and Health Among Deployed Veterans (SHADE) program including participants with a median deployment duration of almost one year and identified burn pit smoke as one of the main deployment-associated inhalation exposures. Burn pit smoke was found to be associated with the prevalence of dyspnea and chronic bronchitis symptoms in veterans [14]. Recently a cohort study by Savitz et al. utilizing the Veterans Health Administration data including almost half a million military personnel, who were followed up after approximately a decade of deployment, identified an association between burn pit smoke exposure and development of asthma, COPD, hypertension, and ischemic stroke [15]. These observations warrant more research on the impact of burn pit smoke on human respiratory health to understand both short- and long-term exposure-associated pulmonary impacts.
Demographic characteristics have already been shown to play a role in deployment-related health outcomes. Data from the SHADE program of the US Veterans Affairs identified an inverse association between cumulative pack-years and pulmonary function (post-bronchodilator FEV1%-predicted and FEV1/FVC%-predicted) among veterans [16]. This association could potentially be aggravated as per the recent report of a tobacco-use epidemic among the US military service members and veterans [17]. Apart from these, other host factors, such as biological sex, are important in understanding the health impact of deployment in veterans. However, studies addressing the health impacts of deployment on women veterans mostly focused on the reproductive and mental health disorders [18]. In spite of the previous reports of disparity in health outcome based on sex and smoking habits [19], very limited conclusive information is available on this topic. Hence susceptibilities associated with demographic features including lifestyle (such as smoking habit) or host factors (including sex) needs to be explored in more detail.
Understanding the health impacts of burn pit smoke exposure is challenging for several reasons. A recent review by Wang et al. has pointed out the issues in deriving a definitive conclusion on the topic [20]. Previously, we reported that smoke condensates from common burn pit waste materials cause adverse pulmonary effects in murine models [21, 22]. Analyses of simulated burn pit smoke condensates indicated the presence of an array of chemical compounds with associated physiological impacts [21, 23]. Furthermore, the waste incineration condition (flaming and smoldering representing differential temperature and modified combustion efficiencies) influenced the levels of chemical components of toxicological interest present in the smoke [21,22,23]. Recently, we used human respiratory cells to evaluate the cellular effects of smoke condensates of common burn pit waste materials [24, 25]. Burn pit smoke condensates caused cytotoxic effects on cultured human respiratory cells, with effects being dependent on waste source material and combustion temperature [25].
In this new study, we further extended our previous experimental approach to pursue the following novel objectives. First, we set out to evaluate gene expression modulation in human airway epithelial cells (HAECs) by smoke condensates from three burn pit waste materials (cardboard, plywood and plastic) incinerated under smoldering and flaming conditions; subsequently, we examined the potential mechanisms of gene expression modulation, by analyzing the association with chemical components present in the smoke condensates. We further determined the impact of HAEC donor’s demographic features, such as smoking history and sex on the smoke condensate-mediated alterations and potential association with any known human diseases. Finally, we characterized the effects of individual smoke condensates on the HAEC-mediated cytokine secretion using a data collapsing approach. Data presented here demonstrate that exposure to burn pit smoke results in significant gene expression changes in HAECs, with increased susceptibility observed in smokers and females, suggesting they may be more affected by burn pit-induced respiratory health issues.
Methods
Culture of human airway epithelial cells
Human lungs deemed unsuitable for transplantation were obtained under the auspices of the University of North Carolina Biomedical Institutional Review Board approved protocol #03-1396. All donors were free of prior chronic lung disease. Authorized representatives provided informed consent for research use. Primary human airway epithelial cells (HAEC) were obtained from the trachea and bronchi as previously described [26]. Demographic details of the twelve donors are provided in Table 1. An equal ratio of biological female and male donors was used to ensure any sex-dependent comparison can be performed. An equal ratio of non-smokers and smokers was also planned to further understand the impact of the donors’ smoking habit on burn pit smoke condensate-mediated modulation of gene expression. Smokers used in the study had an average 20 pack year history. Passage-one cells were thawed and seeded directly on collagen coated 6.5 mm Transwell inserts (Costar #3470). Cells were differentiated at the air-liquid interface (ALI) for 28–35 days until use.
Burn pit smoke condensate generation
The simulated burn pit waste combustion and smoke condensate generation protocol has been published before [21, 25, 27] and is briefly described herein. The current study used three waste materials (military compliant cardboard and plywood, as well as three different types of plastics) incinerated under two conditions, smoldering and flaming. Selection of the materials was based on the composition of Military base waste reported previously by the United States Army Logistics Innovation Agency (USALIA) [28] identifying a major contribution of these three source materials in the overall composition of military waste incinerated in the burn pits. Military-grade cardboard and plywood were obtained from ActionPak, Inc., Bristol, PA, and plastic waste was sourced form Edwards Industrial Surplus, Robards, KY. Plastic waste was a mixture of low-density polyethylene, high-density polyethylene, polyethylene terephthalate, and polystyrene pellets. Waste materials were incinerated in a quartz-tube furnace connected to a multi-stage cryotrap system [27]. Details of the two combustion conditions (smoldering and flaming) have been published before [25]. The combustion condition was based on the modified combustion efficiency (MCE) (ratio of emitted CO2 concentrations to emitted CO2 and CO concentrations (ΔCO2 / (ΔCO2 + ΔCO) x 100); smoldering condition typically indicate 500ºC temperature and an MCE between 65 and 85%. Flaming condition incinerates materials at 640 ºC with an MCE around 95%. Stock condensates were stored at a concentration of 1 mg/ml which were sonicated and vortexed to resuspend the particles, and diluted with secondary stock in phosphate buffered saline (PBS) before being added to the apical surface of the HAECs at a final concentration of 25 µg/cm2. Vehicle control group was treated with sterile PBS.
Exposure of ALI cultures to smoke condensates and sample collection
Smoke condensate exposure was conducted at a final particulate concentration of 25 µg/cm2 as described before [25]. The rationale for selecting the dose has been published before [24]. For the dose calculation, we considered a typical minute ventilation expected to be attained during extensive physiological activities by the deployed veterans within a time span of 24 h. Following an exposure duration of 24 h, epithelial integrity was evaluated using transepithelial electrical resistance (TEER) measurements; apical wash was collected to evaluate lactate dehydrogenase (LDH) activity to ascertain any cytotoxicity caused by the exposure; basolateral media was used to quantitate cytokine secretion using MSD analysis; and cell lysate was collected and used to purify RNA (Ambion PureLink™ RNA kit, 12183025) as described previously [29].
Assessment of cytotoxicity and epithelial integrity
Cytotoxicity and epithelial integrity were evaluated respectively by LDH activity and TEER measurement. Following 24-hour exposure to burn pit smoke condensates at a dose of 25 µg/cm2, apical wash was collected in PBS and cell-free supernatant was obtained by centrifuging at 600 g for 5 min. LDH activity in the apical wash supernatant was quantified using the Roche Cell Cytotoxicity Kit (Cat#11644793001) following the manufacturer’s instructions. An EVOM2 epithelial voltohmmeter (World Precision Instruments) was used to measure TEER as previously described [25] and the data represented as ohms per cm2 of HAEC apical surface area.
Analysis of cytokine secretion and data collapsing approach
A total of 30 candidate proteins were analyzed in the basolateral media following 24 h of exposure to smoke condensates. The candidate proteins belonged to three panels of Meso Scale Discovery multiplex assay corresponding to cytokines, chemokines, and pro-inflammatory panels. Of all tested, 17 mediators were identified with adequate data coverage, defined as having at least 75% of samples showing concentrations above the lowest standard; these 17 mediators were thus selected for further analysis. After this filtration, less than 1.2% of all data was missing and values were imputed using imputeLCMD package in R based on quantile regression imputation of left-censored data (QRILC) [30].
We used a weighted gene co-expression network analysis (WGCNA) as previously described [23, 31, 32] to identify clusters of corelated mediators. The PBS-treated control samples were divided into four demographic groups, namely non-smokers, smokers, female, and male. Using a power of five and minimum module size of three, we identified three clusters (modules) of correlated mediators. To further understand the modulation of these clusters by burn pit smoke condensate, a score for each cytokine was calculated for each exposure. The cytokine score was calculated using a scaling formula [score = (cytokinesample – cytokineminimum)/(cytokinemaximum – cytokineminimum)]. For each exposure, an overall cluster value was calculated by adding the scores of the cytokines for the specific cluster and dividing the value with the number of cytokines included in the cluster. The calculated score for each exposure represented the capacity of the specific condensate to co-modulate the group of cytokines. The log transformed value of the scores were plotted for each exposure.
Library preparation and bulk RNA sequencing
Azenta Life Sciences (South Plainfield, NJ, USA) performed the next generation sequencing for this study. Qubit 4.0 Fluorometer (Life Technologies, Carlsbad, CA, USA) was used for RNA samples quantification and Agilent TapeStation 4200 (Agilent Technologies, Palo Alto, CA, USA) verified RNA integrity. NEBNext Ultra II RNA Library Prep Kit for Illumina was used to prepare RNA sequencing libraries following manufacturer’s instructions (NEB, Ipswich, MA, USA). Briefly, Oligod(T) beads-enriched mRNAs were fragmented at 94°C for 15 minutes. Complementary DNA (cDNA), prepared from RNA molecules fragments were end repaired and adenylated at 3’ ends, followed by ligation of universal adapters; subsequently, index addition and library enrichment by PCR with limited cycles. The sequencing library was validated on the Agilent TapeStation (Agilent Technologies, Palo Alto, CA, USA), and quantified by both Qubit 2.0 Fluorometer (Invitrogen, Carlsbad, CA) and by quantitative PCR (KAPA Biosystems, Wilmington, MA, USA). After clustering of the sequencing libraries on a flowcell, the same was loaded on the Illumina NovaSeq instrument following manufacturer’s instructions. Sequencing was performed using a 2 × 150 bp Paired End (PE) configuration. Control software conducted image analysis and base calling. Sequencer-generated raw sequence data (.bcl files) were converted into fastq files and de-multiplexed using Illumina’s bcl2fastq 2.17 software. Index sequence identification allowed one mismatch. After investigating the quality of the raw data, Trimmomatic v.0.36 was used to trim the sequence reads to eliminate poor quality nucleotides and adapter sequences. STAR aligner v.2.5.2b mapped the trimmed reads to the Homo sapiens GRCh38 reference genome and BAM files were generated from this step. Subread package v.1.5.2 feature Counts was used to calculate unique gene hit counts and unique reads that fell within exon regions were counted.
Determination of differentially expressed genes
The study design included total six smoke condensate exposure groups at a concentration of 25 µg/cm2 in phosphate buffered saline (PBS); smoldering and flaming condensates of three burn pit waste materials, namely cardboard, plywood and plastic. Control cells were treated with PBS. Subsequent analysis was performed using limma-voom framework on [33, 34] R software platform (v 4.3.3) [35]. Limma is an R/Bioconductor package that relies on linear modeling of complex experimental design. Voom function of limma creates a precision weight for observations by estimating the mean-variance relationship of log-transformed count value. Transcripts with expressions in at least 10 groups were included in the analysis. Subsequently, the empirical Bayes analysis pipeline is utilized for further analyses and detection of differentially expressed genes (DEG). Each exposure group (six smoke condensates and control) had 12 donors (equal ratio of female vs. male, and non-smokers vs. smokers). The limma-voom framework utilizes Benjamini–Hochberg method for multiple comparison correction while identifying DEGs. Count data of two technical replicates of each donor for the exposure groups were combined and used for analysis. Voom-transformed data was fitted to a linear model and expression list values were used in the multidimensional scaling plot. Analysis model factored in both exposures and individual donors to account for the donor-to-donor variations. An adjusted p-value of < = 0.1 with absolute log fold change of > = 0.5 was considered significant. Volcano plots were prepared using EnhancedVolcano package [36]; eulerr package was used to create Euler diagrams [37]. Heatmaps were generated using pheatmap package [38]. Pre-ranked (log2 fold change) gene set enrichment analysis (GSEA) and gene ontology (GO) analysis were performed with the Bioconductor R package clusterProfiler [39]. Enrichment map was generated using enrichplot package using top ten enriched pathways [40].
Determination of DEGs for different demographic cohorts
The twelve HAEC cell donors were categorized into four cohorts based on tobacco use history and sex: non-smokers, smokers, female, and male. Demographic details of cohorts are reported in Table 1. For background difference between non-smokers and smokers, and between female and male donors, the modeling involved all exposure groups without any correction for different donors. The control samples from each demographic group were compared and DEGs were used for the heatmap. Multidimensional plotting was used to demonstrate the overall differences between each pair of demographic groups; each sample is shown in the plot showing relative gene expression profiles.
For comparison across different exposure groups within each demographic group (non-smokers, smokers, female, and male), we included the donor’s information as an additional factor in the modeling before identifying the DEGs. For host-factor based analyses, the modeling included separately analyzing the samples corresponding to the demographic group, followed by performing the comprehensive comparison using other tools. For association analysis between DEGs and disease profiles, we referred to the DisGeNET database [41] using the gprofiler2 package [42]. Briefly, the gene matrix transposed file was downloaded from DisGeNET website (accessed on June 13, 2024) and smoke condensate mediated DEGs (combining both up- and down regulated) were used to detect disease conditions significantly associated with the gene list for each demographic group.
Correlation analysis between chemical components and gene expression
Concentration of sixteen EPA priority PAHs and several oxy/nitro PAHs along with several inorganic elements in the burn pit smoke condensates were used to perform a Pearson’s correlation analysis with the summed count of the DEGs significantly altered in all flaming condensates to detect positive and negative correlation using the R package psych [43] and result plotted using corrplot [44] without multiple comparison correction. A p < = 0.05 was considered significant for the Pearson’s correlation analysis, and positive and negative correlation has been identified as respectively blue and red dots in the plot.
Statistical analysis
We used the Kruskal Wallis test to compare across the exposure groups for the TEER and LDH data. For the comparison of cytokine levels in basolateral media (MSD analysis), we used rstatix package (version 0.7.2) in R environment [45]. Friedman’s test was used to evaluate overall differences across all exposure groups for each cytokine using normalized cytokine levels. For pairwise comparison, we used Dunn’s test. A p-value of 0.05 was considered significant for Kruskal Wallis and Friedman’s tests. An adjusted p-value of 0.05 was considered significant in Dunn’s test in pairwise comparison.
Results
Cellular viability was not compromised following exposure
Burn pit smoke condensates at higher doses (> 50 µg/cm2) cause adverse cellular impacts in human airway epithelial cells (HAEC) [25]. To explore the impact of burn pit smoke condensate exposure gene expression and cytokine secretion from HAECs, we exposed air-liquid interface cultures of HAECs from twelve donors (Table 1) to a sub-lethal dose (25 µg/cm2) of smoke condensates from cardboard, plywood, and plastic source materials, generated under smoldering and flaming conditions, as described in the methods and reported previously [25]. Cells from equal number of donors from both sexes (female and male) were included, combining cells from both non-smokers and smokers (Table 1).
Following exposure to the burn pit smoke condensates, we first determined the viability of the cultures by measuring apical lactate dehydrogenase (LDH) secretion and transepithelial electrical resistance (TEER) of the monolayers, as surrogates of cytotoxicity detection. Previously we demonstrated that burn pit smoke condensates at the dose of 25 µg/cm2 were not cytotoxic, and cell viability was not compromised following the exposure at this dose. Similar to our previous observation, current exposure at 25 µg/cm2 did not cause any significant alteration in terms of apical LDH secretion and TEER measurements, compared to PBS-treated control group (Figure S1). These observations confirmed that the exposures used here did not cause overt cytotoxicity.
Plywood flaming condensate most effectively altered gene expression
Following exposure for 24 h to smoke condensates, we collected the cell lysate and isolated RNA to understand the potential impact of the burn pit smoke condensates on the HAEC transcriptome. Of the six condensates tested (smoldering and flaming condensates of cardboard, plywood, and plastic), flaming condensates consistently resulted in more significant DEGs compared to their smoldering counterparts (Fig. 1). The elevated numbers of significant DEGs in flaming exposure groups indicate more intensified impact of these condensates compared to smoldering ones (Fig. 1 and Table S1). Of the three waste materials tested, plywood caused the most alterations (total 329) in the HAEC transcriptome, resulting in significant up- and down- regulation of respectively 93 and 236 genes (Fig. 1 and Table S1). Flaming condensates of plastic and cardboard caused respectively 101 and 92 significant DEGs; of these DEGs, plastic flaming condensate caused 66 up- and 27 down-regulated genes (Fig. 1 and Table S1). For cardboard flaming condensate, the numbers of up- and down-regulated DEGs were 64 and 38, respectively (Fig. 1 and Table S1).
Flaming condensates caused more DEGs than smoldering ones. HAEC ALI cultures were exposed to flaming and smoldering condensates from cardboard (a), plywood (b), and plastic (c) wastes for 24 h, and modulation of gene expression was evaluated by bulk RNA sequencing. The volcano plot for each exposure group shows log2 fold changes on x-axis and log10 adjusted p-values on y-axis. Dots in the volcano plot indicate individual genes, with red color indicating significant DEGs (absolute log2FC > = 0.5 and adjusted p-value < = 0.1). Genes with the highest fold change are identified within the plot. Euler diagram comparing total significant DEGs from flaming and smoldering condensates of each waste material is shown
Compared to the flaming condensates, smoldering condensates caused fewer significant DEGs with total number for plywood, plastic and cardboard being 7, 16 and 38, respectively (Figure S2 and Table S1). Unlike flaming condensates, the proportion of significantly up- and down-regulated DEGs were almost equal for all smoldering condensates.
Pathway analysis of differentially expressed genes and the role of waste source materials
Since flaming condensates resulted in the most DEGs, we investigated any potential overlap across the three-waste materials to alter gene expression profiles. Figure 2A shows that while condensates from flaming plywood smoke caused the largest number of changes, 47 genes were commonly affected by all flaming condensates (Fig. 2a). Of these 47 genes, 18 and 29 genes were down- and up-regulated, respectively, by all flaming condensates (Fig. 2b). Gene Ontology (GO) enrichment analysis revealed that these DEGs were mostly associated with immune cell migration (leukocyte aggregation, leukocyte migration and neutrophil chemotaxis) and detoxification (toxin metabolic process, epoxygenase P450 pathway and secondary metabolic process) (Fig. 2c).
Flaming condensate modulated DEGs and pathways. DEGs from flaming condensate exposure groups were further analyzed to identify the most potent source material and commonality in gene expression modulation. Euler plot (a) showing plywood flaming condensate resulted in the most DEGs compared to cardboard and plastic. (b) Heatmap of the 47 common DEGs from all flaming condensates showing relative levels for each condensate obtained by adding the RNA-seq count values and scaling for each gene: 18 and 29 genes being respectively down- and up-resulted by all flaming condensates. Pathway analysis (c) of the 47 common DEGS exhibited impact on immune cell migration and detoxification. The color of the scale in panel B denotes relative expression level of each gene across the four groups. Scale in panel C denotes adjusted p-value
Pathway analysis of differentially expressed genes and the role of waste source materials
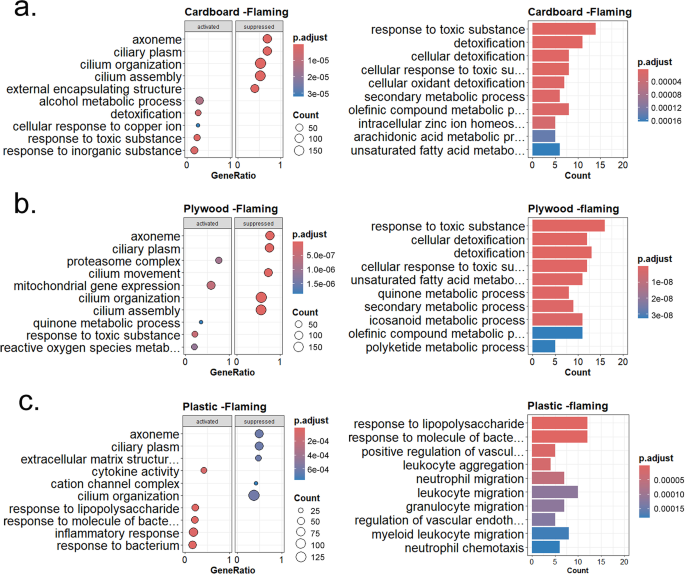
To understand the overall impact of individual waste materials on cellular functions, we performed gene set enrichment analysis (GSEA) and Gene Ontology (GO) term enrichment analyses. Figure 3 shows GSEA and GO analyses for each waste material under the flaming condition. As indicated in Fig. 3a, cardboard flaming waste exposure altered several cellular processes and molecular functions, including impairment of cilium assembly and functions and enhancement of detoxification pathways. Interestingly, genes associated with response to copper and zinc ions were elevated in HAECs exposed to cardboard flaming smoke condensate, which agrees with our previous study reporting that cardboard flaming condensate contained high levels of both copper and zinc metals [21]. When plywood flaming condensate mediated DEGs were examined by GSEA and GO enrichment analyses, similar suppression of multiciliated cell function and enhancement of detoxification pathways were noted (Fig. 3b). Several pathways associated with detoxification processes and response to reactive oxygen species were elevated in the HAECs following exposure to plywood flaming condensate.
GSEA, GO and enrichment plot of significant DEGs from flaming smoke condensate exposure. Differentially expressed genes from flaming condensate exposure groups were used to perform GSEA and GO enrichment analysis. The pairwise similarity matrix of top ten categories from GSEA is shown as the enrichment plot. The top five activated and suppressed pathways from GSEA and ten GO enrichment categories are shown as dot plot and bar diagram respectively, for cardboard (a), plywood (b), and plastic (c). The color of the scale denotes adjusted p-value, and the size of circles indicates the number of genes
In contrast to cardboard and plywood, plastic flaming condensate mostly altered genes related to leukocyte aggregation and migration. Neutrophil and granulocyte migration, and vascular endothelial growth factor (VEGF) formation dominated the DEGs created by plastic flaming condensate (Fig. 3c). The enrichment map of pairwise similarities of the enriched terms indicated that plastic flaming condensate caused activation of inflammatory processes and cellular death pathways in HAECs (Figure S3c); on the other hand, the enrichment map for plywood and cardboard flaming smoke condensate exposure groups emphasized ciliary assembly and functions (Figures S3a-b).
The smoldering combustion condition yields a reduced chemical load, as indicated by the lower levels of inorganic elements and PAHs in these condensates, compared to flaming ones [21, 23]. Accordingly, we detected a lower number of significant DEGs from the condensates generated under smoldering conditions of the three waste materials tested in the study (Figure S2). Cardboard smoldering condensate caused activation of oxidoreductase activity, especially related to pyridine cofactor nicotinamide adenine dinucleotide (Figure S4). Enrichment of pathways associated with the cell xenobiotic response were noted, with suppression ribosomal structure and subunit assembly associated genes. Plywood smoldering condensate also caused activation of oxidoreductase activity with suppression of genes associated with cell division and cell organelle lumen. In contrast, plastic smoldering condensate mediated changes in biosynthetic processes and ion (zinc and copper) regulation.
Differential gene expression is regulated by chemical components of smoke condensates
We previously detected the presence of 16 polycyclic aromatic hydrocarbons (PAHs) designated as priority pollutants by the US Environmental Protection Agency (EPA), along with several oxy/nitro PAHs and a range of inorganic elements in simulated burn pit smoke condensates [21]. To elucidate the potential mechanisms of burn pit smoke-mediated differential gene expression, we performed a correlation analysis of the 47 genes commonly modulated by the flaming condensates (Fig. 2a) with the 16 priority PAHs and 9 oxy/nitro PAHs, as described in the Methods section using Pearson’s correlation test without multiple comparison correction to perform an aggregate level of analysis. As depicted in Fig. 4, the common DEGs exhibited strong positive and negative correlation with multiple PAHs. In our present study, IL6 expression was positively modulated by all priority PAHs and six oxy/nitro PAHs (Fig. 4a). Of the 16 priority PAHs, fluoranthene, pyrene, benz(a)anthracene, chrysene, benzo(b)fluoranthene and benzo(k)fluoranthene positively and negatively modulated several DEGs. Interestingly, oxy/nitro PAHs showed strong correlation with more genes, both positively and negatively impacting the expression; as shown in Fig. 4a, apart from CRCT1, HSPA6, and CYP2A13, all other genes were either positively or negatively associated with one of four oxy/nitro PAHs (namely 9,10-anthraquinone, 1,8-naphthalic anhydride, benzanthrone and 1-pyrenecarboxaldehyde), suggesting broad impact on differential gene expression by these chemical components.
Correlation analysis between chemical components and flaming condensate induced DEGs. Pearson’s test was used to perform correlation between chemical compounds present in the condensates and the common DEGs from flaming condensate as described in the Methods section. Strong positive and negative correlation were noted for several PAHs and metals with DEGs
When the levels of inorganic elements present in smoke condensates were correlated with the 47 genes, aluminum, molybdenum, and silicon were found to be positively and negatively correlated with multiple genes (Fig. 4b). Interestingly, RASD1, ALDH3A1, VIPR1, ITGB7, CRCT1, HSPA6, CYSRT1 and RPTN genes were positively correlated with multiple elements. In contrast, VIM, SLC5A1, IGFBP5, PPP1R3C, ABI3BP, STC1, PTPRT, SCNN1G, MAB21L3 were negatively correlated with three or more elements. Although IL6 expression was positively correlated with most of the PAHs, it was only positively correlated with antimony.
Differences between demographic cohorts based on tobacco use and sex
Individual demographic attributes play a significant role in determining susceptibility to disease development and health outcomes. To understand the potential role of sex and smoking history on burn pit smoke exposure-mediated pulmonary outcome, we first determined the differences in constitutive gene expression between non-smoker and smoker donors, and between female and male donors, as detailed in the Methods section. To do so, we compared the gene expression in vehicle-treated cells from the groups and determined change in gene expression pattern between the groups. Significant DEGs comparing non-smokers with smokers, and female and male donors are reported in table S2. As shown in Figure S5a, multidimensional scaling (MDS) plot of non-smokers and smokers’ samples did not indicate any specific clustering. A total of seven significant DEGs were detected. Importantly, gamma ENaC subunit expression was higher in smokers (Figure S5b), indicating that smokers may be susceptible to increased airway dehydration.
Previous studies have reported different gene expression in airway cells from females and males [46, 47]. When gene expression pattern in HAECs from female and male donors was compared, the MDS plot clearly indicated clustering of the samples based on sex with minimal overlap (Fig. 5a). We identified a total of 59 significant DEGs between HAECs from female and male donors, 9 and 50 of these genes were respectively up- and down-regulated in female donors, compared to the male donors (Fig. 5b). Interestingly several of these DEGs have already been reported by other groups. As expected, the long noncoding RNA X-linked X-inactive-specific transcript (XIST), which plays a critical role in X-chromosome inactivation in females [48], was elevated in female donors compared to male donors (Fig. 5a). Similar to previous reports [46, 47], ZFX, RPS4X and KDM6A were also increased in female donors. Conversely, RPS4Y1, KDM5D, USP9Y and DDX3Y were elevated in male donors, as previously reported [49]. Several genes coding for kinesin superfamily proteins (KIFs: KIF11, KIF2C, KIFC1, KIF4A and KIF20A), cyclin protein coding cell cycle-related genes (CCNA2, CCNB1, and CCNB2) and ubiquitin-conjugating enzyme genes (UBE2S, UBE2T, and UBE2C) were elevated in the male donors. Sex chromosome-encoded RNA helicases DDX3X and DDX3Y were respectively elevated in female and male donors (Fig. 5b).
Background differences in gene expression profiles between biological female and male donors. Baseline gene expression pattern between female and male donors were distinctly different, as identified in the multidimensional scaling (MDS) plot (a). A total 59 DEGs were noted between female and male; heatmap (b) showing the normalized level of each gene in individual donor including 9 up- and 50 down-regulated genes in female donors. The top ten pathways activated and suppressed in female donors (c), as identified by GSEA, are shown as a dot plot
GSEA and GO enrichment analysis were utilized to understand the differences between female and male donors. As shown in Fig. 5c, genes associated with vascular structure were activated in HAECs from female donors; on the other hand, genes related to multiciliated cell structure and function were suppressed in female donors. The GO enrichment pathways in female and male donors’ cells are shown as Figure E6 indicating categories significantly associated with the DEGs.
Demographic characteristics and the effects of simulated burn pit smoke condensate exposure
To evaluate any donor demographic-based differential impact of burn pit smoke condensate, we estimated the significant DEGs separately among cells derived from non-smokers, smokers, female, and male donors (six in each cohort). When flaming condensate derived significant DEGs were considered in total, smokers cells exhibited more alterations compared to non-smoker donor cells (Fig. 6a, Tables S3 and S4). Following exposure to flaming condensates cells from donors without any reported smoking history showed a reduced number of DEGs compared to HAECs from donors with a smoking history (Fig. 6a). For cardboard flaming condensate exposure, these cells showed a total of 155 DEGs (Table S4; 81 up and 74 down) compared to 52 DEGs (Table S3; 27 up and 25 down) in HAECs from non-smoking donors. When plastic flaming smoke condensate mediated DEGs were compared between non-smokers and smokers, cells from the latter exhibited more DEGs (Table S4, total 148; 100 up and 48 down) compared to the former group (Table S3, total 30; 16 up and 14 down). As noted earlier, plywood flaming condensate exposure results in the highest numbers of significant DEGs; HAECs from donors with smoking history showed 484 DEGs (Table S4; 133 up and 351 down) compared to HAECs from non-smoking donors (Table S3, total 225; 68 up and 157 down).
Distinct role of the host factors flaming burn pit smoke condensate exposure. Donors were separated based on the smoking history (non-smokers and smokers) and biological sex (female and male) and Euler diagrams were generated using significant DEGs by flaming smoke condensate was evaluated as described in the Methods. (a) All three flaming condensates caused more alterations in cells from donors with history of smoking than non-smoker donors. Comparison based on sex (b) demonstrated that female donors’ cells were more responsive to flaming condensate exposure than male donors’ cells
Comparison based on the sex of the donor following exposure to flaming condensates showed more alterations in HAECs from female donors than male donors (Fig. 6b). Cardboard flaming condensate caused 139 (Table S5; 70 up and 69 down) and 43 (Table S6; 29 up and 14 down) DEGs in respectively HAECs from female and male donors. Plastic flaming condensate also caused more DEGs in HAECs from female donors with a total number of significant DEGs of 147 (Table S5; 90 up and 57 down) than HAECs from male donors (Table S6, total 32; 21 up and 11 down). Flaming condensate of plywood waste caused a total 459 DEGs (Table S5; 132 up and 327 down) in HAECs from female donors versus a total of 225 in cells from male donors (Table S6, 69 up and 156 down).
Compared to the flaming condition, smoldering burn pit smoke condensates caused lower numbers of DEGs (Figure S7); hence, a demographics-based comparison was mostly inconclusive for these exposure groups. The only impact of donor’s smoking history on condensate derived DEGs was noted for cardboard smoldering condensate exposure, where HAECs from donors with smoking history showed a total 81 DEGs (Table S4; 33 up and 48 down) compared to cells from non-smokers (Table S3, total 20; 3 up and 17 down). A marginally elevated number of DEGs was noted in cells from non-smokers following plastic condensate exposure (Table S3, total 24; 4 up and 20 down) as compared to HAECs from donors with a smoking history (Table S4, total 17; 15 up and 2 down). Sex-based comparison of DEGs from smoldering condensates only showed more DEGs in HAECs from male donors (Table S6, total 58; 12 up and 46 down) following cardboard condensate exposure than HAECs from female donors (Table S5, total 42; 22 up and 20 down).
To understand the susceptibility of the four demographic cohorts towards burn pit smoke exposure-derived diseases, we used significant DEGs from plywood flaming smoke condensate exposure group to perform enrichment analysis, utilizing a gene-disease association database from DisGeNET [41]. Both up- and down-regulated genes were used to perform enrichment analysis of disease gene signatures. Table S7 reports the results of enrichment analysis, including significantly associated diseases in the four demographic cohorts. Figure S8 demonstrates the human diseases associated with the four demographic cohorts. All of the cohorts showed a significant association with contact dermatitis, contact hypersensitivity, and cancer of different organs. When focusing on respiratory diseases, HAECs from non-smokers exhibited association with asbestosis and asbestos exposure-derived pulmonary fibrosis. Alterations in gene expression in HAECs from donors with a smoking history were closely associated with asthma, bronchiectasis, and emphysema. When sex-dependent associations were examined, female donors were associated with asthma, several types of neoplasm and carcinoma.
Co-modulation of cytokine secretion by smoke condensates
We have previously shown that exposure to burn pit smoke condensate causes modulation of cytokine secretion by HAECs [25]. When the basolateral media of the HAECs was analyzed for the secretion of cytokines, we observed that eleven (IP-10, IL-7, IFN-g, MCP-1, IL-1b, Eotaxin-3, IL-1a, IL-12p70, GM-CSF, IL-6, VEGF-A) of the total seventeen cytokines were significantly altered by condensate exposures compared to vehicle control (Table S8). However, pairwise comparison did not identify many significant differences (Table S8). The relative levels of each cytokine across the different exposure groups are shown as a heatmap (Fig. 7a). To further emphasize the co-modulation of the cytokines by burn pit smoke condensates, we used data collapsing approaches to identify clusters of mediators that are likely co-modulated, similar to our previous studies [32]. The vehicle control group is divided into cohorts representing samples from four demographic groups: non-smokers, smokers, female and male. Three clusters of co-modulating cytokine mediators were detected, which included respectively seven, six and four mediators (Fig. 7b). Cluster 1 included most chemokines (Eotaxin-3, MCP-1 and TARC), gamma-chain cytokines (IL-2, and IL-7), IL-10 and VEGF-A; cluster two included six mediators (IL-1α, IL-1β, IFN-γ, IL-8, IL-6, and IL-12p70), including mostly pro-inflammatory cytokines; cluster three included four mediators (GM-CSF, IL-13, IP-10, and TNF-α) with both pro- and anti-inflammatory properties. When individual cluster modulation was considered, plywood flaming condensate caused the most suppression of clusters one and three, with the highest activation of cluster two (Fig. 7c). Flaming condensate of cardboard induced cytokines belonging to the cluster one. These observations suggest that burn pit smoke condensate-mediated modulation of cytokine secretion by HAECs is source material and combustion temperature dependent, and plywood flaming smoke condensate induces the highest immunomodulatory/inflammatory response in HAECs.
Co-modulation of cytokines by smoke condensates. Basolateral media was collected following burn pit smoke condensate exposure and HAEC-secreted mediators were quantified by MSD analysis. Seventeen mediators were further analyzed using WGCNA method and grouped into three clusters (a) based on co-modulation in control samples. Relative impact of smoke condensates on each cluster was evaluated as described in the Methods; bar graph (b) shows the relative score representing modulation of each cluster by the condensates
Discussion
In 2011, the National Academy of Sciences’ Institute of Medicine reported increased PM and acrolein exposure of deployed military personnel from burning of waste, and cautioned about a potential risk of respiratory and cardiovascular diseases in the veterans [4]. The PACT (Sergeant First Class Heath Robinson Honoring Our Promise to Address Comprehensive Toxics) act, which was signed into law in 2022, expands benefits to the US veterans exposed to burn pit smoke and other toxic compounds, and presumes more than twenty medical conditions are associated with deployment-related exposures [50]. However, mechanisms or causality of deployment-associated pulmonary diseases are still largely unknown. In the present study, we explored the impact of simulated burn pit smoke condensate exposure on gene expression profiles and cytokine release from HAECs to elucidate the potential impact of such exposure on human airways. Emphasis was given to burn pit waste source material, combustion conditions (i.e. incineration temperature) and demographic characteristics of the exposed individuals. We observed that flaming smoke condensate consistently showed heightened impact on HAEC gene expression as compared to smoldering ones, with plywood being the most potent source material. When demographic characteristics were considered, tobacco use history and female sex were found to be associated with intensified impact on HAEC gene expression from burn pit smoke.
Burn pit waste composition is complex and highly heterogeneous; hence, smoke exposure-mediated respiratory impact likely varies depending on the source material and the combustion temperature. Additionally, wide variation in the ambient particulate concentration expected to prevail in the overseas military bases may vary widely depending on several circumstances [51]. To address this issue, we tested three source materials (military-grade cardboard and plywood, as well as plastics) incinerated under two conditions, smoldering (lower temperature) and flaming (higher temperature) which generate lower and higher concentrations of harmful chemicals respectively at a sublethal particulate dose of the smoke condensates [21, 23]. We have previously reported that burn pit smoke mixtures generated under flaming conditions induced greater cytotoxicity in HAECs [25]. In our current study, smoke condensate from plywood material combusted at flaming condition exhibited the greatest impact on HAEC gene expression. As flaming combustion conditions generate higher levels of chemical components compared to smoldering conditions, especially those with known respiratory toxicity [21, 23], this is not unexpected. Further pathway analysis revealed transcriptomic similarity in HAECs exposed to cardboard and plywood smoke condensate, but less so for cells exposed to plastic smoke condensate. Both cardboard and plywood source materials at the flaming condition influenced detoxification response pathways in HAECs. In contrast, plastic smoke condensate at this condition impacted mostly anti-microbial and immunomodulatory pathways. In our previous study, we observed distinct differences in terms of chemical composition across these three waste source materials [21, 23]. PAH concentrations were higher in plastic smoke condensates which potentially modified gene expression associated with immunomodulatory pathways in our current study. In contrast, inorganic elements enriched in cardboard and plywood smoke condensates induced gene expression changes associated with detoxification responses. These observations agree with previous reports linking PAHs to immunomodulatory responses [52] and inorganic elements to toxicity [53]. Of note, the plywood and cardboard used to generate the smoke condensates were military grade packaging material presumably heat- and chemical-treated, which is different from similar material used for regular shipping or construction. Since combustion of these modified products generate more chemicals [54], this may contribute towards the cellular impacts noted in our study. The toxicity derived from combustion of military-grade plywood is of particular concern, since these materials typically constitute a significant portion of a typical burn pit waste [21]. Furthermore, as these chemicals likely co-occur in the military burn pit smoke mixtures, long-term exposure of these chemicals during military deployment may lead to development of adverse health outcomes, including lung diseases, cancer, autoimmune and allergic diseases [52, 55].
To further examine these links between smoke mixture chemicals and gene expression modulation in HAECs, we conducted a correlation analysis of the chemicals presents in the smoke and the flaming smoke condensate mediated common DEGs identified in HAECs. These results identified the role of polyaromatic hydrocarbons and some inorganic elements in driving specific gene expression changes. Specifically, IL6 expression was found to be positively associated with PAHs, but not metals. Previous studies have detected PAH-mediated modulation of IL6 expression in human [56, 57], animal model [58] and in vitro cell culture systems [59] The strongest correlations were noted for oxy-PAHs present in the smoke condensates. Incidentally, oxy-PAHs are far less studied than nitro-PAHs or PAHs in general. In vitro studies have identified mutagenic and cytotoxic effects of oxy-PAHs in human cells [60, 61]. Misaki et al. previously reported tumor-promoting activity by several PAHs and oxy/nitro-PAHs in mouse cells along with induction of CYP1A1 [62], which is associated with aryl hydrocarbon receptor (AhR) activation and lung cancer [63]. Activation of AhR is also associated with asthma and COPD [64]. In our study, we observed a significant positive correlation between CYP1A1 gene expression and several oxy-PAHs, indicating a potential mechanism towards pulmonary disease pathogenesis in burn pit smoke exposed individuals.
We further examined the association of burn pit smoke condensate-mediated gene expression modulation with human disease signatures. Differential regulation of genes in HAECs exposed to plywood flaming smoke condensate, which exhibited the highest changes, demonstrated association with potential diseases/health symptoms in the four demographic cohorts of our study. DEGs in smokers’ and female donors’ HAECs showed resemblance with several diseases already reported in the deployed Veterans, indicating susceptibility of these demographic cohorts towards development of burn pit smoke exposure-derived health impacts. Especially, resemblance with asthma gene signature, which is a frequently reported post-deployment health symptom [4, 65], was detected in HAECs from smokers and female donors. Contact dermatitis and contact hypersensitivity were associated with all four demographic groups. A significant association was also noted for HAECs from smokers with gene signature of pulmonary emphysema and bronchiectasis, suggesting that burn pit smoke exposure may be a contributing factor to these smoking-induced diseases. Apart from these, resemblance with several neoplasm and carcinoma-related gene profiles were noted in all four demographic cohorts, which has already been speculated before [66, 67].
Regulation of cytokine release is a complex, multifaceted process, with involvement of mutually dependent signaling cascades [68]. Co-expression and co-modulation of cytokines have been reported for several diseases, indicating a necessity to study their expression as groups, rather than individually [32, 69]. We emphasized co-modulation of cytokines by identifying clusters of mediators across the different exposures. Cytokine expression in control samples exhibited co-modulation of three clusters; cluster 1 included chemokines (Eotaxin-3, MCP-1 and TARC), angiogenic VEGF-A, and interleukins − 2, -7 and − 10; cluster 2 included pro-inflammatory interleukins − 1α, -1β, -12p70, -6, -8, and IFN-γ; GM-CSF, IL-13, IP-10 and TNF-α constituted cluster 3. Plywood flaming condensate exerted the highest impact in suppressing clusters 1 and 3, simultaneously inducing cytokines of cluster 2, which included several pro-inflammatory candidates. These observations bear critical importance in terms of burn pit smoke-derived health effects. Cytokines play an important role in asthma and other pulmonary diseases, which are frequently reported by Veterans post-deployment; higher levels of the cluster 2 cytokines IL-1α, IL-1β [70], IL-8 [71], IL-6 [72] and IFN-γ [73] are associated with asthma severity. Apart from these, IL-1β, IL-8, IL-6 also been implicated in emphysema and bronchiectasis [74]. Interestingly, plywood flaming smoke mediated significant DEGs exhibited association with asthma, bronchiectasis, and emphysema. Together, these observations provide a mechanistic basis linking exposure to plywood flaming smoke and asthma symptoms in deployed veterans.
As expected, smoldering smoke condensates exhibited fewer changes in gene expression than flaming condition at the same mass concentration of exposure and corresponding to their lower amounts of metals and PAHs. Of the three source materials from smoldering conditions smoke condensate of cardboard caused the highest alterations in gene expression. Ubiquitin C-terminal hydrolase-L1 (UCHL1), which plays an important role in lung cancer pathogenesis [75], was up-regulated by all smoldering smoke condensates. Although the effect on HAEC gene expression was lower from smoldering smoke condensates, pathway analysis echoed similar patterns as flaming condensates, showing similarity between cardboard and plywood with plastic-derived impact being markedly unique. Cardboard and plywood showed activation of genes corresponding to metabolic and detoxification pathways with suppression of genes important in intracellular processes. In contrast, plastic smoldering smoke condensate activated genes related to biosynthetic pathways simultaneously suppressing metal homeostasis processes.
Our study observations can be extended to understand and address health effects of other common environmental pollution scenarios originating from waste incineration. For example, according to the estimates from the Organization for Economic Cooperation and Development (OECD), approximately 19% of plastics are incinerated with emission of harmful chemicals in the atmosphere [76]. Hence, the gene expression modulation in HAECs observed from the plastic waste smoke condensate exposure may contribute towards understanding the pulmonary effects of smoke from plastic waste incineration. Combustion of demolition and construction wood typically generates toxic emissions including PCDD/Fs and biphenyls (PCBs) [77]. Modulation of gene expression from plywood, cardboard, and plastic smoke condensates may also represent impact of ambient smoke exposures, such as wildfire smoke, as these mixtures increasingly include emissions from burning anthropogenic sources (plastics, plywood, metals, etc.), especially at the wildland urban interface (WUI), “the zone of transition between unoccupied land and human development” [78].
Our current observations identified critical insights into the potential impact of burn pit smoke exposure on pulmonary health. However, our study has some limitations. First, although ethnicity has been suggested as a critical factor in determining susceptibility to tobacco smoke-induced decline in lung function [79], this donor demographic aspect was beyond the scope of our study. Second, we have examined three specific representative waste materials at two combustion conditions, not considering the highly complex and diverse composition and conditions of the typical burn pit waste. In fact, a dose-dependent study design and analysis may provide more information, indicating the differential regulation of cellular pathways in response to smoke condensate exposure. Third, our study design includes a 24-hour exposure regime at a single dose; given the dynamic composition of burn pit waste, in addition to the widely variable incineration condition expected in the real-world scenario, it is indeed challenging to create a convincing model to examine the short- and long-term health effects of burn pit smoke inhalation. However, a repeated chronic exposure strategy with doses encompassing both lower and high concentrations is needed to fully understand the burn pit smoke-mediated health effects. Finally, our observations on different demographic cohorts need to be interpreted cautiously, as we have only six donors per cohort. Of note, a statistical power calculation [80] based on our data indicated that the sample size of n = 6 is sufficient to identify group-based differences, similar to previous publications [81, 82]. However, this was only true at FDR = 0.1 and not at FDR = 0.05. The demographic characteristics dependent altered gene expression profile noted here may correspond to both adverse health effect vulnerability and enhanced protection in these specific cohorts. Future studies with greater sample size will enhance the sensitivity of uncovering group-based differences in responses elicited by simulated burn pit smoke exposures.
Conclusions
We have performed a comprehensive study analyzing the effect of simulated smoke condensate from three burn pit waste materials incinerated under two conditions on HAECs, focusing on the modulation of gene expression and cytokine secretion. Effects on genes modulated by all smoke condensate exposures were associated with oxy-PAH levels in the mixtures, suggesting that regardless of the burn pit waste material, generation of oxy-PAH is a potential driving factor of adverse health effects. Exposures to plywood smoke condensate generated under flaming conditions had the most significant effects on gene expression changes and immunomodulation with effects being more abundant in HAECs from smokers and females. Hence, tobacco use, and biological sex may play an important role in response to burn pit smoke inhalation and in determining the susceptibility towards adverse health impacts. Importantly, we determined that the gene expression modulation by burn pit waste smoke condensates resemble those associated with known pulmonary and other diseases’ profiles, with several of these have already been listed as presumable deployment-derived diseases in the PACT law. Additionally, association of gene modulation with the diseases of other organs indicates the potential systemic adverse health effects of burn pit smoke exposure, which needs to be explored further.
Data availability
The raw data of the study has been deposited in the University of North Carolina -Center for Environmental Medicine, Asthma, and Lung Biology Dataverse. All scripts associated with data analysis of the current study are available through the Github page.
Abbreviations
- HAEC:
-
Human airway epithelial cells
- ALI:
-
Air-liquid interface
- PM:
-
Particulate material
- TEER:
-
Transepithelial electrical resistance
- LDH:
-
Lactate dehydrogenase
- PAHs:
-
Polyaromatic hydrocarbons
- COPD:
-
Chronic obstructive pulmonary disease
References
Garshick E, Abraham JH, Baird CP, Ciminera P, Downey GP, Falvo MJ, et al. Respiratory health after military service in Southwest Asia and Afghanistan. An official American thoracic Society Workshop Report. Ann Am Thorac Soc. 2019;16 8:e1–16. https://doi.org/10.1513/AnnalsATS.201904-344WS. https://www.ncbi.nlm.nih.gov/pubmed/31368802.
Review of the department of defense enhanced particulate matter surveillance program report. Washington (DC); 2010.
Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East–Part 1: ambient sampling. Inhal Toxicol. 2009;21 4:297–326. https://doi.org/10.1080/08958370802464273. https://www.ncbi.nlm.nih.gov/pubmed/19235610.
Institute of Medicine. Long-term health consequences of exposure to burn pits in Iraq and Afghanistan. Washington, DC: National Academies; 2011.
Krefft SD, Rose CS, Nawaz S, Miller YE. Deployment-related Lung disorders. Fed Pract. 2015;32 6:32–8. https://www.ncbi.nlm.nih.gov/pubmed/30766070.
Pugh MJ, Jaramillo CA, Leung KW, Faverio P, Fleming N, Mortensen E, et al. Increasing prevalence of chronic lung disease in veterans of the wars in Iraq and Afghanistan. Mil Med. 2016;181 5:476–81. https://doi.org/10.7205/MILMED-D-15-00035. https://www.ncbi.nlm.nih.gov/pubmed/27136656.
Slatore CG, Falvo MJ, Nugent S, Carlson K. Afghanistan and Iraq war veterans: mental health diagnoses are associated with respiratory disease diagnoses. Mil Med. 2018;183:5–6. https://doi.org/10.1093/milmed/usx108. https://www.ncbi.nlm.nih.gov/pubmed/29420832. :e249-e57.
Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31 5:67–71; https://doi.org/10.2500/aap.2010.31.3383. https://www.ncbi.nlm.nih.gov/pubmed/20929596
US-EPA: Current Open Burning / Open Detonation Facilities. https://www.epa.gov/hwpermitting/current-open-burning-open-detonation-facilities (2024). Accessed August 12 2024.
UNEP. Global waste management outlook 2024: beyond an age of waste, turning rubbish into a resource. 2024. https://www.unep.org/resources/global-waste-management-outlook-2024
Vinti G, Bauza V, Clasen T, Medlicott K, Tudor T, Zurbrugg C, et al. Municipal Solid Waste Management and adverse Health outcomes: a systematic review. Int J Environ Res Public Health. 2021;18:8. https://doi.org/10.3390/ijerph18084331. https://www.ncbi.nlm.nih.gov/pubmed/33921868.
Mattiello A, Chiodini P, Bianco E, Forgione N, Flammia I, Gallo C, et al. Health effects associated with the disposal of solid waste in landfills and incinerators in populations living in surrounding areas: a systematic review. Int J Public Health. 2013;58 5:725–35. https://doi.org/10.1007/s00038-013-0496-8. https://www.ncbi.nlm.nih.gov/pubmed/23887611.
Ashworth DC, Elliott P, Toledano MB. Waste incineration and adverse birth and neonatal outcomes: a systematic review. Environ Int. 2014;69:120–32. https://doi.org/10.1016/j.envint.2014.04.003. https://www.ncbi.nlm.nih.gov/pubmed/24831282.
Garshick E, Redlich CA, Korpak A, Timmons AK, Smith NL, Nakayama K, et al. Chronic respiratory symptoms following deployment-related occupational and environmental exposures among US veterans. Occup Environ Med. 2024;81 2:59–65. https://doi.org/10.1136/oemed-2023-109146. https://www.ncbi.nlm.nih.gov/pubmed/37968126.
Savitz DA, Woskie SR, Bello A, Gaither R, Gasper J, Jiang L, et al. Deployment to Military Bases with Open burn pits and Respiratory and Cardiovascular Disease. JAMA Netw Open. 2024;7 4:e247629. https://doi.org/10.1001/jamanetworkopen.2024.7629. https://www.ncbi.nlm.nih.gov/pubmed/38662371.
Maccarone JR, Sterns OR, Timmons A, Korpak AM, Smith NL, Nakayama KS, et al. Deployment-related cigarette smoking behaviors and pulmonary function among U.S. Veterans. Mil Med. 2024. https://doi.org/10.1093/milmed/usae049. https://www.ncbi.nlm.nih.gov/pubmed/38536226.
Lang AE, Melzer AC, Good CB, Upson DJ. Recommendations for addressing the Tobacco and Nicotine Use Epidemic in U.S. Military Service members and veterans. Ann Am Thorac Soc. 2023;20 9:1229–32. https://doi.org/10.1513/AnnalsATS.202302-177VP. https://www.ncbi.nlm.nih.gov/pubmed/37289723.
Batuman F, Bean-Mayberry B, Goldzweig C, Huang C, Miake-Lye IM, Washington DL et al. Health effects of military service on women veterans. 2011.
Haghani A, Arpawong TE, Kim JK, Lewinger JP, Finch CE, Crimmins E. Female vulnerability to the effects of smoking on health outcomes in older people. PLoS ONE. 2020;15 6:e0234015. https://doi.org/10.1371/journal.pone.0234015. https://www.ncbi.nlm.nih.gov/pubmed/32497122.
Wang X, Doherty TA, James C. Military burn pit exposure and airway disease: implications for our veteran population. Ann Allergy Asthma Immunol. 2023;131 6:720–5. https://doi.org/10.1016/j.anai.2023.06.012. https://www.ncbi.nlm.nih.gov/pubmed/37343826.
Kim YH, Warren SH, Kooter I, Williams WC, George IJ, Vance SA, et al. Chemistry, lung toxicity and mutagenicity of burn pit smoke-related particulate matter. Part Fibre Toxicol. 2021;18. https://doi.org/10.1186/s12989-021-00435-w. 1:45. https://www.ncbi.nlm.nih.gov/pubmed/34915899.
Vance SA, Kim YH, George IJ, Dye JA, Williams WC, Schladweiler MJ, et al. Contributions of particulate and gas phases of simulated burn pit smoke exposures to impairment of respiratory function. Inhal Toxicol. 2023;35. https://doi.org/10.1080/08958378.2023.2169416. 5–6:129 – 38; doi:. https://www.ncbi.nlm.nih.gov/pubmed/36692431.
Kim YH, Rager JE, Jaspers I, Gilmour MI. Computational Approach to Link chemicals in anthropogenic smoke particulate matter with toxicity. Chem Res Toxicol. 2022;35 12:2210–3. https://doi.org/10.1021/acs.chemrestox.2c00270. https://www.ncbi.nlm.nih.gov/pubmed/36373932.
Rogers K, WaMaina E, Barber A, Masood S, Love C, Kim YH, et al. Emissions from plastic incineration induce inflammation, oxidative stress, and impaired bioenergetics in primary human respiratory epithelial cells. Toxicol Sci. 2024. https://doi.org/10.1093/toxsci/kfae038. https://www.ncbi.nlm.nih.gov/pubmed/38539046.
Ghosh A, Payton A, Gallant SC, Rogers KL Jr., Mascenik T, Hickman E, et al. Burn pit smoke condensate-mediated toxicity in human airway epithelial cells. Chem Res Toxicol. 2024;37 5:791–803. https://doi.org/10.1021/acs.chemrestox.4c00064. https://www.ncbi.nlm.nih.gov/pubmed/38652897.
Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol. 2013;945:109–21. https://www.ncbi.nlm.nih.gov/pubmed/23097104. doi: 10.1007/978-1-62703-125-7_8.
Kim YH, Warren SH, Krantz QT, King C, Jaskot R, Preston WT, et al. Mutagenicity and lung toxicity of smoldering vs. flaming emissions from various biomass fuels: implications for Health effects from Wildland fires. Environ Health Perspect. 2018;126(1:017011). https://doi.org/10.1289/EHP2200. https://www.ncbi.nlm.nih.gov/pubmed/29373863.
VA USALIAFB. USARCENT AOR Contingency Base Waste Stream Analysis. An Analysis of Solid Waste Streams at Five Bases in the US Army Central (USARCENT) Area of Responsibility. 2013.
Brocke SA, Billings GT, Taft-Benz S, Alexis NE, Heise MT, Jaspers I. Woodsmoke particle exposure prior to SARS-CoV-2 infection alters antiviral response gene expression in human nasal epithelial cells in a sex-dependent manner. Am J Physiol Lung Cell Mol Physiol. 2022;322 3:L479–94. https://doi.org/10.1152/ajplung.00362.2021. https://www.ncbi.nlm.nih.gov/pubmed/35107034.
imputeLCMD. A Collection of Methods for Left-Censored Missing Data Imputation. https://cran.r-project.org/web/packages/imputeLCMD/imputeLCMD.pdf
Rager JE, Clark J, Eaves LA, Avula V, Niehoff NM, Kim YH, et al. Mixtures modeling identifies chemical inducers versus repressors of toxicity associated with wildfire smoke. Sci Total Environ. 2021;775:145759. https://doi.org/10.1016/j.scitotenv.2021.145759. https://www.ncbi.nlm.nih.gov/pubmed/33611182.
Rebuli ME, Stanley Lee A, Nurhussien L, Tahir UA, Sun WY, Kimple AJ, et al. Nasal biomarkers of immune function differ based on smoking and respiratory disease status. Physiol Rep. 2023;11 3:e15528. https://doi.org/10.14814/phy2.15528. https://www.ncbi.nlm.nih.gov/pubmed/36780897.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43 7:e47. https://doi.org/10.1093/nar/gkv007. https://www.ncbi.nlm.nih.gov/pubmed/25605792.
Law CW, Chen Y, Shi W, Smyth GK. Voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15(2):R29. https://doi.org/10.1186/gb-2014-15-2-r29. https://www.ncbi.nlm.nih.gov/pubmed/24485249.
R Core Team. (2024). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. (2024).
Blighe K, Rana S, Lewis M. EnhancedVolcano: publication-ready volcano plots with enhanced colouring and labeling. R Package Version 1 22 0. 2023. https://doi.org/10.18129/B9.bioc.EnhancedVolcano.
Larsson J, Gustafsson P. A case study in fitting area-proportional euler diagrams with ellipses using eulerr. In: SetVR@ diagrams. 2018. pp. 84–91.
pheatmap. Pretty Heatmaps. https://CRAN.R-project.org/package=pheatmap.
Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innov (Camb). 2021;2 3:100141. https://doi.org/10.1016/j.xinn.2021.100141. https://www.ncbi.nlm.nih.gov/pubmed/34557778.
Yu G, enrichplot. Visualization of Functional Enrichment Result. R package version 1.22.0. 2023; https://doi.org/10.18129/B9.bioc.enrichplot
Pinero J, Ramirez-Anguita JM, Sauch-Pitarch J, Ronzano F, Centeno E, Sanz F, et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48. https://doi.org/10.1093/nar/gkz1021. https://www.ncbi.nlm.nih.gov/pubmed/31680165. D1:D845-D55.
Kolberg L, Raudvere U, Kuzmin I, Vilo J, Peterson H. gprofiler2 -- an R package for gene list functional enrichment analysis and namespace conversion toolset g:Profiler. F1000Res. 2020;9. https://doi.org/10.12688/f1000research.24956.2. https://www.ncbi.nlm.nih.gov/pubmed/33564394.
psych package v2.4.1. https://rdocumentation.org/packages/psych/versions/2.4.1
Wei T, Simko V. R package ‘corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. https://github.com/taiyun/corrplot
rstatix. Pipe-Friendly Framework for Basic Statistical Tests. https://rpkgs.datanovia.com/rstatix/
Lopes-Ramos CM, Chen CY, Kuijjer ML, Paulson JN, Sonawane AR, Fagny M, et al. Sex differences in Gene Expression and Regulatory Networks across 29 human tissues. Cell Rep. 2020;31 12:107795. https://doi.org/10.1016/j.celrep.2020.107795. https://www.ncbi.nlm.nih.gov/pubmed/32579922.
Gershoni M, Pietrokovski S. The landscape of sex-differential transcriptome and its consequent selection in human adults. BMC Biol. 2017;15(1):7. https://doi.org/10.1186/s12915-017-0352-z. https://www.ncbi.nlm.nih.gov/pubmed/28173793.
Loda A, Heard E. Xist RNA in action: past, present, and future. PLoS Genet. 2019;15 9:e1008333. https://doi.org/10.1371/journal.pgen.1008333. https://www.ncbi.nlm.nih.gov/pubmed/31537017.
Wapeesittipan P, Joshi A. Integrated analysis of robust sex-biased gene signatures in human brain. Biol Sex Differ. 2023;14 1:36. https://doi.org/10.1186/s13293-023-00515-w. https://www.ncbi.nlm.nih.gov/pubmed/37221602.
Richmond BW, Miller RF. The honoring our PACT Act: an improved commitment to veterans’ health. Ann Am Thorac Soc. 2023;20 4:508–9. https://doi.org/10.1513/AnnalsATS.202208-718VP. https://www.ncbi.nlm.nih.gov/pubmed/36410012.
Butler DA, Styka AN, Savitz DA, editors. Assessment of the Department of Veterans Affairs Airborne Hazards and Open Burn Pit Registry. Washington (DC) 2017; 2017.
Yu YY, Jin H, Lu Q. Effect of polycyclic aromatic hydrocarbons on immunity. J Transl Autoimmun. 2022;5:100177. https://doi.org/10.1016/j.jtauto.2022.100177. https://www.ncbi.nlm.nih.gov/pubmed/36561540.
Kozlowski H, Kolkowska P, Watly J, Krzywoszynska K, Potocki S. General aspects of metal toxicity. Curr Med Chem. 2014;21 33:3721–40. https://doi.org/10.2174/0929867321666140716093838. https://www.ncbi.nlm.nih.gov/pubmed/25039781.
Hansen-Bruhn I, Hull TRJF. Smoke Toxic fire Protecting Timber Treatments. 2023;141:103977.
Nemery B. Metal toxicity and the respiratory tract. Eur Respir J. 1990;3 2:202–19. https://www.ncbi.nlm.nih.gov/pubmed/2178966.
Wang H, Liu J, Kong Q, Li L, Gao J, Fang L, et al. Cytotoxicity and inflammatory effects in human bronchial epithelial cells induced by polycyclic aromatic hydrocarbons mixture. J Appl Toxicol. 2021;41 11:1803–15. https://doi.org/10.1002/jat.4164. https://www.ncbi.nlm.nih.gov/pubmed/33782999.
Ye J, Zhu R, He X, Feng Y, Yang L, Zhu X, et al. Association of plasma IL-6 and Hsp70 with HRV at different levels of PAHs metabolites. PLoS ONE. 2014;9 4:e92964. https://doi.org/10.1371/journal.pone.0092964. https://www.ncbi.nlm.nih.gov/pubmed/24722336.
Rojas GA, Saavedra N, Saavedra K, Hevia M, Morales C, Lanas F, et al. Polycyclic aromatic hydrocarbons (PAHs) exposure triggers inflammation and endothelial dysfunction in BALB/c mice: a pilot study. Toxics. 2022;10:9. https://doi.org/10.3390/toxics10090497. https://www.ncbi.nlm.nih.gov/pubmed/36136462.
Totlandsdal AI, Ovrevik J, Cochran RE, Herseth JI, Bolling AK, Lag M, et al. The occurrence of polycyclic aromatic hydrocarbons and their derivatives and the proinflammatory potential of fractionated extracts of diesel exhaust and wood smoke particles. J Environ Sci Health Tox Hazard Subst Environ Eng. 2014;49 4:383–96. https://www.ncbi.nlm.nih.gov/pubmed/24345236.
McCarrick S, Cunha V, Zapletal O, Vondracek J, Dreij K. In vitro and in vivo genotoxicity of oxygenated polycyclic aromatic hydrocarbons. Environ Pollut. 2019;246:678–87. https://doi.org/10.1016/j.envpol.2018.12.092. https://www.ncbi.nlm.nih.gov/pubmed/30616058.
Clerge A, Le Goff J, Brotin E, Abeillard E, Vaudorne I, Denoyelle C, et al. In vitro genotoxicity potential investigation of 7 oxy-PAHs. Environ Mol Mutagen. 2023;64 3:176–86. https://doi.org/10.1002/em.22531. https://www.ncbi.nlm.nih.gov/pubmed/36757094.
Misaki K, Takamura-Enya T, Ogawa H, Takamori K, Yanagida M. Tumour-promoting activity of polycyclic aromatic hydrocarbons and their oxygenated or nitrated derivatives. Mutagenesis. 2016;31(2):205–13. https://doi.org/10.1093/mutage/gev076. https://www.ncbi.nlm.nih.gov/pubmed/26656082.
Holme JA, Vondracek J, Machala M, Lagadic-Gossmann D, Vogel CFA, Le Ferrec E, et al. Lung cancer associated with combustion particles and fine particulate matter (PM(2.5)) - the roles of polycyclic aromatic hydrocarbons (PAHs) and the aryl hydrocarbon receptor (AhR). Biochem Pharmacol. 2023;216:115801. https://doi.org/10.1016/j.bcp.2023.115801. https://www.ncbi.nlm.nih.gov/pubmed/37696458.
Chiba T, Chihara J, Furue M. Role of the Arylhydrocarbon receptor (AhR) in the Pathology of Asthma and COPD. J Allergy (Cairo). 2012;2012:372384. https://doi.org/10.1155/2012/372384. https://www.ncbi.nlm.nih.gov/pubmed/22500183.
Abraham JH, Eick-Cost A, Clark LL, Hu Z, Baird CP, DeFraites R, et al. A retrospective cohort study of military deployment and postdeployment medical encounters for respiratory conditions. Mil Med. 2014;179 5:540–6. https://doi.org/10.7205/MILMED-D-13-00443. https://www.ncbi.nlm.nih.gov/pubmed/24806499.
Sharkey JM, Abraham JH. Evaluation of postdeployment cancers among active Duty Military Personnel. US Army Med Dep J. 2015:68–75. https://www.ncbi.nlm.nih.gov/pubmed/26276948
Bith-Melander P, Ratliff J, Poisson C, Jindal C, Ming Choi Y, Efird JT. Slow Burns: a qualitative study of burn pit and toxic exposures among military veterans serving in Afghanistan, Iraq and throughout the Middle East. Ann Psychiatry Clin Neurosci. 2021;4:1. https://www.ncbi.nlm.nih.gov/pubmed/35128459.
Polley DJ, Latham P, Choi MY, Buhler KA, Fritzler MJ, Fritzler ML. Identification of novel clusters of co-expressing cytokines in a diagnostic cytokine multiplex test. Front Immunol. 2023;14:1223817. https://doi.org/10.3389/fimmu.2023.1223817. https://www.ncbi.nlm.nih.gov/pubmed/37600813.
Wiemken TL, Kelley RR, Fernandez-Botran R, Mattingly WA, Arnold FW, Furmanek SP, et al. Using cluster analysis of cytokines to identify patterns of inflammation in hospitalized patients with community-acquired pneumonia: a pilot study. Univ Louisville J Respir Infect. 2017;1 1:3–11. https://doi.org/10.18297/jri/vol1/iss1/1/. https://www.ncbi.nlm.nih.gov/pubmed/28393141.
Osei ET, Brandsma CA, Timens W, Heijink IH, Hackett TL. Current perspectives on the role of interleukin-1 signalling in the pathogenesis of asthma and COPD. Eur Respir J. 2020;55(2). https://doi.org/10.1183/13993003.00563-2019. https://www.ncbi.nlm.nih.gov/pubmed/31727692.
Zhang J, Bai C. Elevated serum Interleukin-8 level as a preferable biomarker for identifying uncontrolled asthma and glucocorticosteroid responsiveness. Tanaffos. 2017;16 4:260–9. https://www.ncbi.nlm.nih.gov/pubmed/29849682.
Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci. 2012;8 9:1281–90. https://doi.org/10.7150/ijbs.4874. https://www.ncbi.nlm.nih.gov/pubmed/23136556.
Figueiredo CA, Rodrigues LC, Alcantara-Neves NM, Cooper PJ, Amorim LD, Silva NB, et al. Does IFN-gamma play a role on the pathogenesis of non-atopic asthma in Latin America children? Allergy Asthma Clin Immunol. 2012;8 1:18. https://doi.org/10.1186/1710-1492-8-18. https://www.ncbi.nlm.nih.gov/pubmed/23253516.
Garth J, Barnes JW, Krick S. Targeting Cytokines as Evolving Treatment Strategies in Chronic Inflammatory Airway Diseases. Int J Mol Sci. 2018;19 11; https://doi.org/10.3390/ijms19113402. https://www.ncbi.nlm.nih.gov/pubmed/30380761
Carolan BJ, Heguy A, Harvey BG, Leopold PL, Ferris B, Crystal RG. Up-regulation of expression of the ubiquitin carboxyl-terminal hydrolase L1 gene in human airway epithelium of cigarette smokers. Cancer Res. 2006;66 22:10729–40. https://doi.org/10.1158/0008-5472.CAN-06-2224. https://www.ncbi.nlm.nih.gov/pubmed/17108109.
Co-operation OfE. Development. Global plastics outlook: policy scenarios to 2060. OECD Publishing; 2022.
Edo M, Ortuño N, Persson P-E, Conesa JA, Jansson SJC. Emissions of toxic pollutants from co-combustion of demolition and construction wood and household waste fuel blends. Chemosphere. 2018;203:506–13.
What is the WUI?. https://www.usfa.fema.gov/wui/what-is-the-wui.html Accessed August 16 2024.
Dransfield MT, Davis JJ, Gerald LB, Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med. 2006;100 6:1110–6. https://doi.org/10.1016/j.rmed.2005.09.019. https://www.ncbi.nlm.nih.gov/pubmed/16236491.
Zhao S, Li CI, Guo Y, Sheng Q, Shyr Y. BMC Bioinformatics. 2018;19(1:191). https://doi.org/10.1186/s12859-018-2191-5. https://www.ncbi.nlm.nih.gov/pubmed/29843589. RnaSeqSampleSize: real data based sample size estimation for RNA sequencing.
Conesa A, Madrigal P, Tarazona S, Gomez-Cabrero D, Cervera A, McPherson A, et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016;17:13. https://doi.org/10.1186/s13059-016-0881-8. https://www.ncbi.nlm.nih.gov/pubmed/26813401.
Li D, Zand MS, Dye TD, Goniewicz ML, Rahman I, Xie Z. An evaluation of RNA-seq differential analysis methods. PLoS ONE. 2022;17 9:e0264246. https://doi.org/10.1371/journal.pone.0264246. https://www.ncbi.nlm.nih.gov/pubmed/36112652.
UNC-CEMALB Dataverse. https://dataverse.unc.edu/dataverse/cemalb
UNC-CEMALB Github. https://github.com/UNC-CEMALB
Acknowledgements
The authors thank Dr. Janice Dye for her careful review of this manuscript. Authors also thank Dr. Meghan Rebuli, Charlotte Love and Kevin Schichlein for their support. The study was funded by FY17 Peer Reviewed Medical Research Program (Award No. W81XWH-18-1-0731) of the United States Department of Defense (I.J.), the U.S. Environmental Protection Agency’s intramural research program of the Office of Research and Development (M.I.G.), and National Institute of Environmental Health Sciences’ grant (R03ES032539) (Y.H.K.). The National Institute of Health’s grant DK065988 and Cystic Fibrosis Foundation’s grant BOUCHE19R0 supports the Tissue Procurement and Cell Culture Core at the Marsico Lung Institute, which provided cells and media for the study. The research described in this article has been reviewed by the Center for Public Health and Environmental Assessment, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views or the policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Funding
Supported by US Department of Defense FY17 Peer Reviewed Medical Research Program W81XWH-18-1-0731 (I.J.), U.S. Environmental Protection Agency’s intramural research program of the Office of Research and Development (M.I.G.), National Institute of Environmental Health Sciences’ grant R03ES032539 (Y.H.K.) and T32 ES001726 (K.L.R), National Institute of Health’s grant DK065988 and Cystic Fibrosis Foundation’s grant BOUCHE19R0.
Author information
Authors and Affiliations
Contributions
AG acquired, analyzed, and interpreted data, and drafted and edited manuscript. KLR and SCG acquired and analyzed data. YHK and JER acquired and analyzed data and edited manuscript. MIG and SHR co-designed the project, obtained funding, and edited the manuscript. IJ co-designed the project, obtained funding, analyzed and interpreted data and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In accordance with the Declaration of Helsinki and under the auspices of the University of North Carolina Biomedical Institutional Review Board approved protocol #03-1396, human lungs deemed unsuitable for transplantation were obtained for the isolation of the human airway epithelial cells used here.
Consent for publication
All authors have approved the manuscript for submission.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ghosh, A., Rogers, K.L., Gallant, S.C. et al. Effects of simulated smoke condensate generated from combustion of selected military burn pit contents on human airway epithelial cells. Part Fibre Toxicol 21, 41 (2024). https://doi.org/10.1186/s12989-024-00604-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12989-024-00604-7